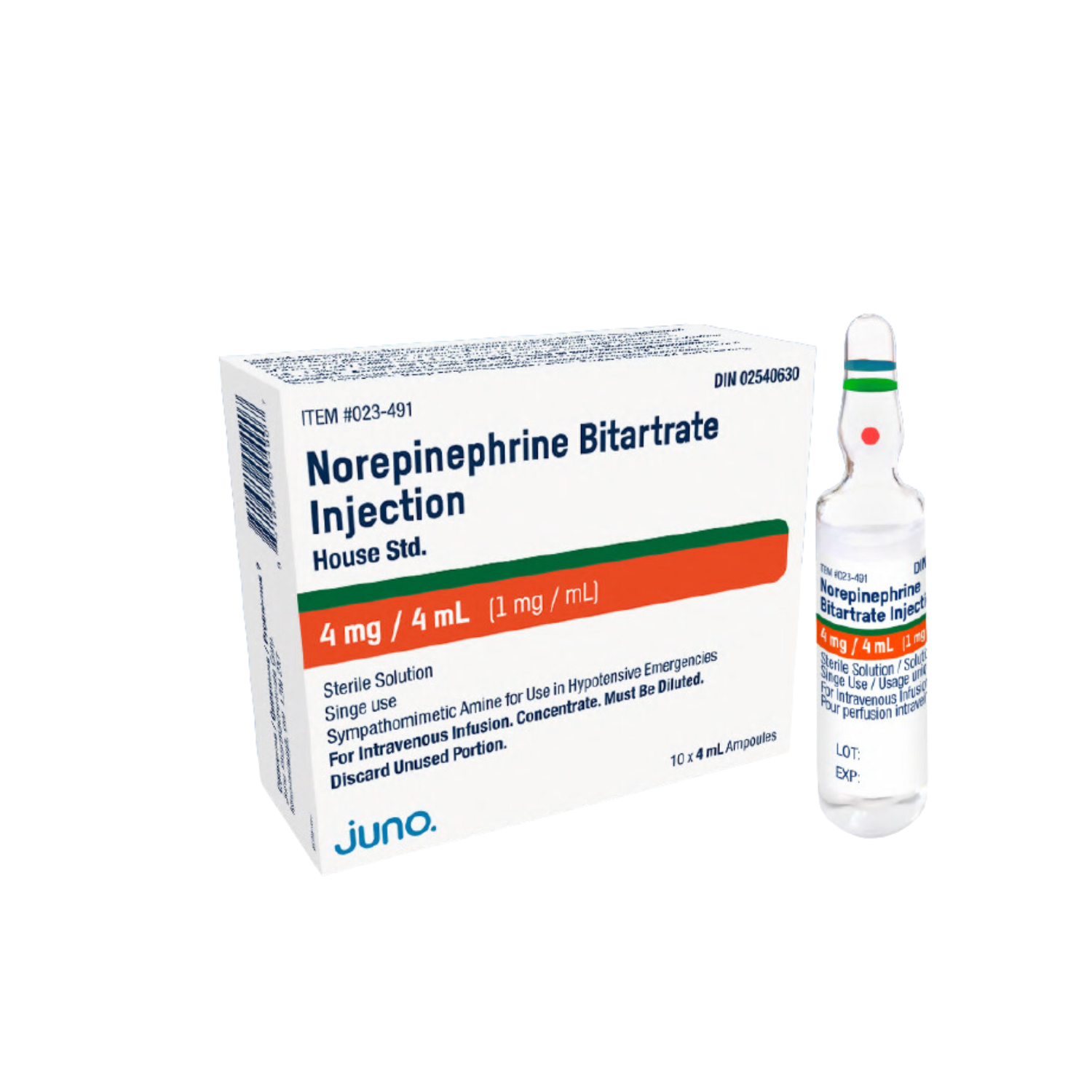
Noromby & Noromby HP New Switching Strategy
Expanded Biosimilar Drug Programs in Saskatchewan, Newfoundland, and Labrador
We are excited to share that our biosimilar drug, Noromby, will now be covered as part of the expanded biosimilar drug programs in Saskatchewan, Newfoundland, and Labrador. As of March 31, 2024, Noromby will replace the current biologic branded product with our more affordable alternative medicine. This initiative will bring cost savings and further aid in the sustainability of our healthcare system, while also ensuring patients have access to safe and effective medication. We are proud to be part of the Biosimilars Initiative, which supports patient access to public drug coverage and new drug benefits.
.